Alcohol Chromic Acid Test
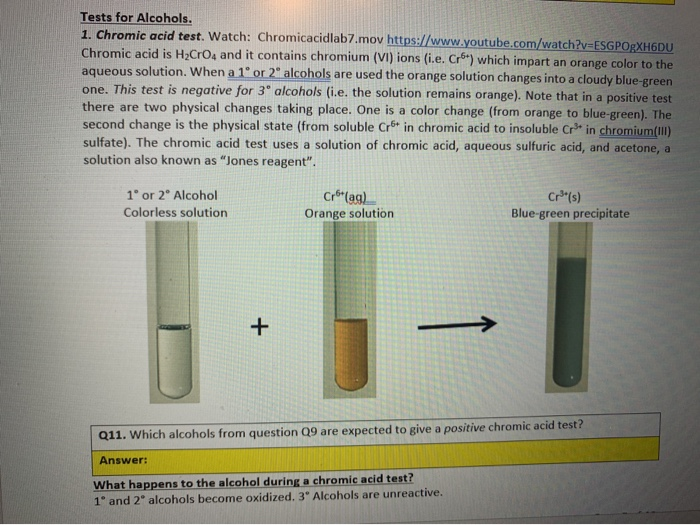
A positive test results in the formation of a blue-green solution from the brown-red color of chromic acid. The chromic acid test for primary and secondary alcohols exploits the resistance of tertiary alcohols to oxidation.

Chromic Acid Test For Alcohols Youtube
Used to distinguish between water-soluble primary secondary and tertiary alcohols with white precipitate.
. Shows positive test for. How to perform the test. Anhydrous Zinc Chloride in concentrated.
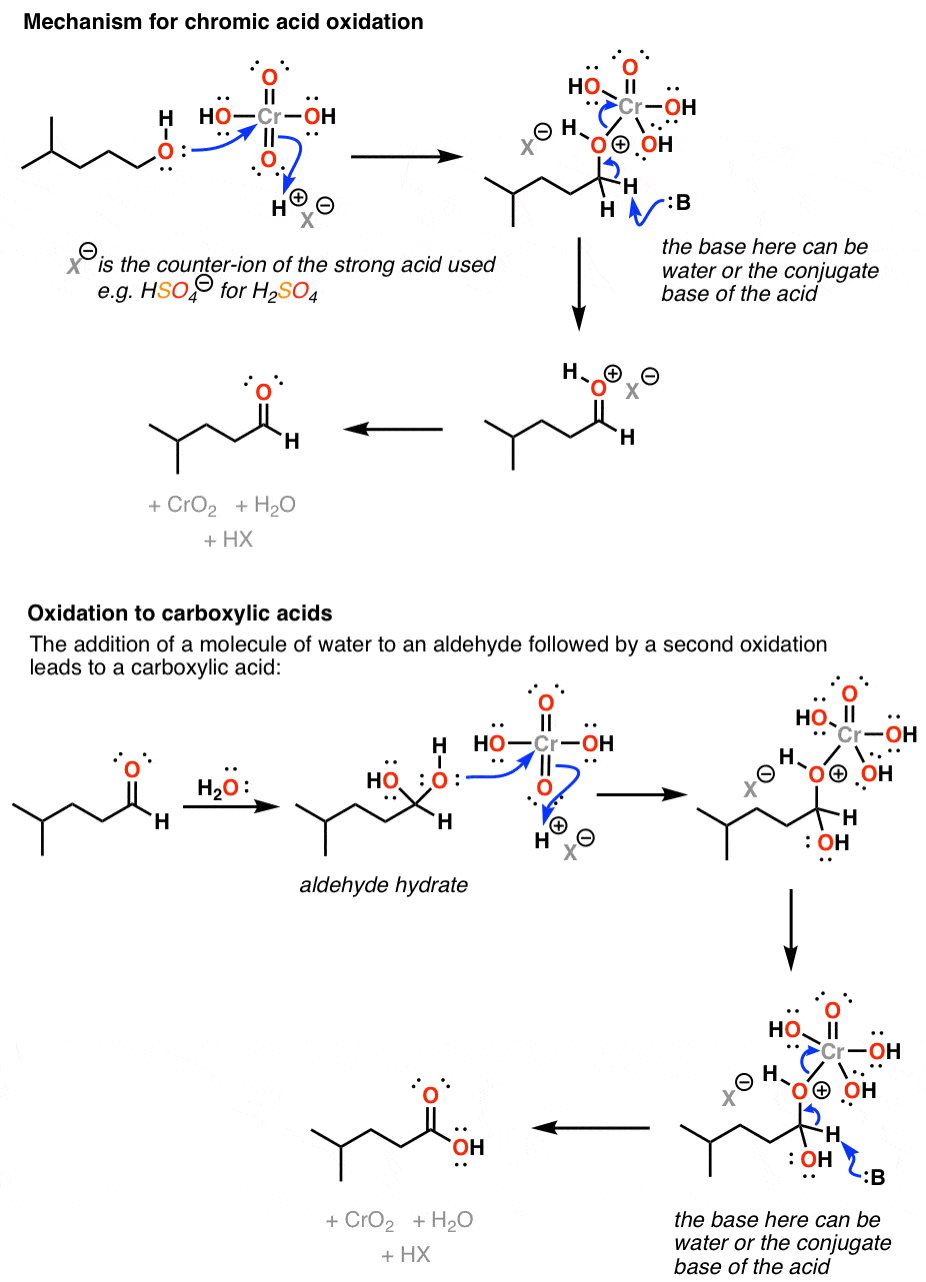
Chromic acid H 2 CrO 4 is a strong acid and a reagent for oxidizing alcohols to ketones and carboxylic acids. Three drops of the compound to be tested are mixed with 5. Place slides in 40 ml 1.
Chromic Acid Test for Alcohols and Aldehydes. This video provides a demonstration in showing alcohols and aldehydes oxidizes under this test to eventually a carboxylic acid under this test. Materials and Methods Materials 1.
Terms in this set 19 Lucas test. Chromic acid solution is strongly acidic and will burn the skin severely. Chromic Acid Oxidation This test distinguishes primary and secondary alcohols from tertiary.
The chromic acid test uses the Jones reactant to test for aldehydes and alcohols. For fairly mundane reasons owing primarily to safety and. When a primary or secondary alcohol is added to the chromic acid.
Dilute with large volumes. Chromic acid will oxidize a primary alcohol first to an aldehyde and then to a. The hydrogen chloride gas reacts with ammonium.
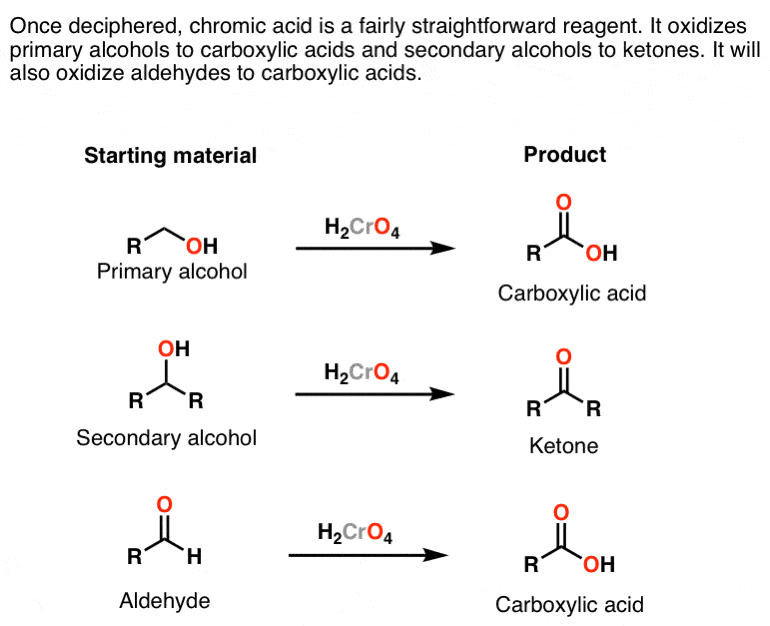
The chromic acid test for primary and secondary alcohols exploits the. Aldehydes and primary alcohols are oxidized to carboxylic acids while the Cr 6 ion in the chromic acid is reduced to. Secondary alcohols are oxidized to ketones while the Cr 6 ion in the chromic acid is reduced to Cr 3.
Three drops of the. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Negative a and positive b results for the chromic acid test.
Reaction of a primary alcohol secondary alcohol and aldehyde with the chromic acid. The Jones reactant has a source of chromium 6 but when it oxidizes aldehydes and alcohols. Acetyl Chloride Test.
Chromic Acid 10 B-Good Chromic Acid 30 B-Good. Alcohols react with acid halides to form esters with the liberation of hydrogen chloride gas. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
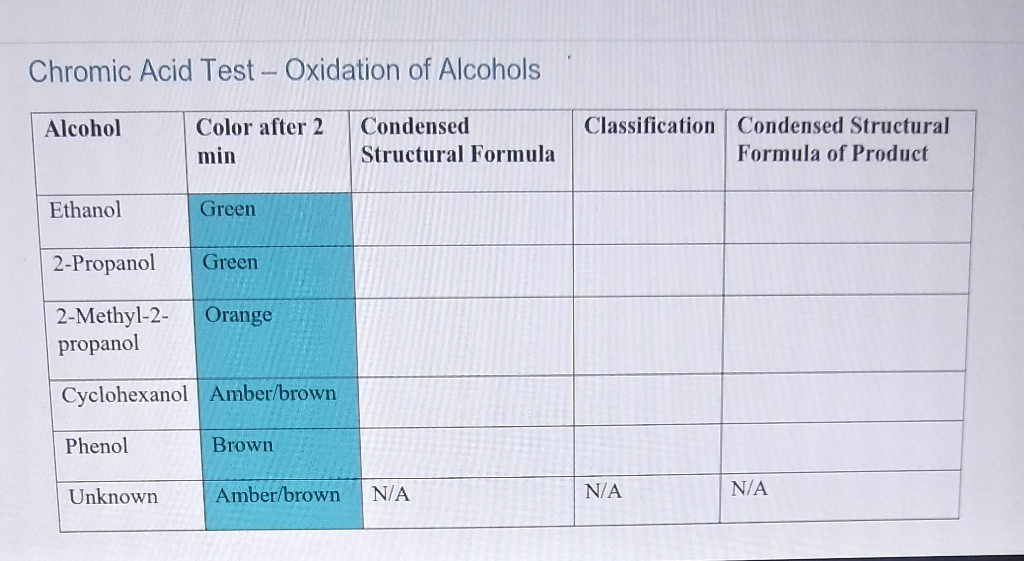
Because of the oxidation is signaled by a color change from orange to a blue-green chromic acid is used as a qualitative analytical test. 1-butanol Primary Alcohol 2-butanol Secondary Alcohol t-butanol Tertiary Alcohol 2. Chromic Acid Test - Oxidation of Alcohols Alcohol Color after 2 min Condensed Structural Formula Classification Condensed Structural Formula of Product Ethanol.
Now lets have a look at this test for alcohols and aldehydes which involves this chemical Oxidation Mechanism Procedure. Tertiary alcohol groups are unaffected. 1 o and 2 o alcohols and aldehydes.
Reagent Friday Chromic Acid H2cro4 Master Organic Chemistry
Reagent Friday Chromic Acid H2cro4 Master Organic Chemistry
Solved Chromic Acid Test Oxidation Of Alcohols Alcohol Chegg Com

Tests For Alcohols 1 Chromic Acid Test Watch Chegg Com
Chem 211 Tests For Aldehydes And Ketones
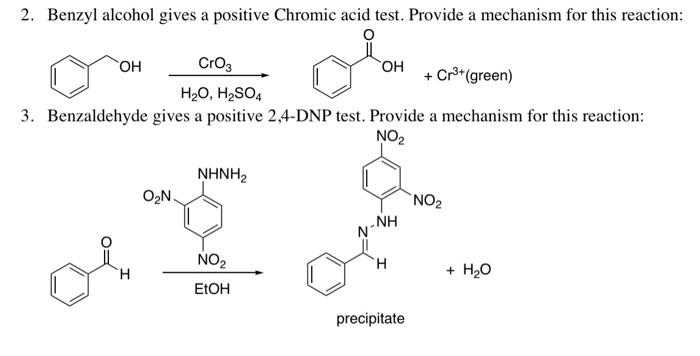
Solved 2 Benzyl Alcohol Gives A Positive Chromic Acid Test Chegg Com
Comments
Post a Comment